Getting Started
Submit a ticket to the Office of Research through JIRA requesting access to Cayuse. Provide your full name and lab group. Note if you would like to schedule an optional virtual training session.
The IACUC Office will create a Cayuse account and provide you with your Username and Password
- Password can be changed after logging into Cayuse
Adding new users is a function of the IACUC Office only.
External collaborators and non-Chapman personnel, can also use Cayuse.
Protocol Review Process
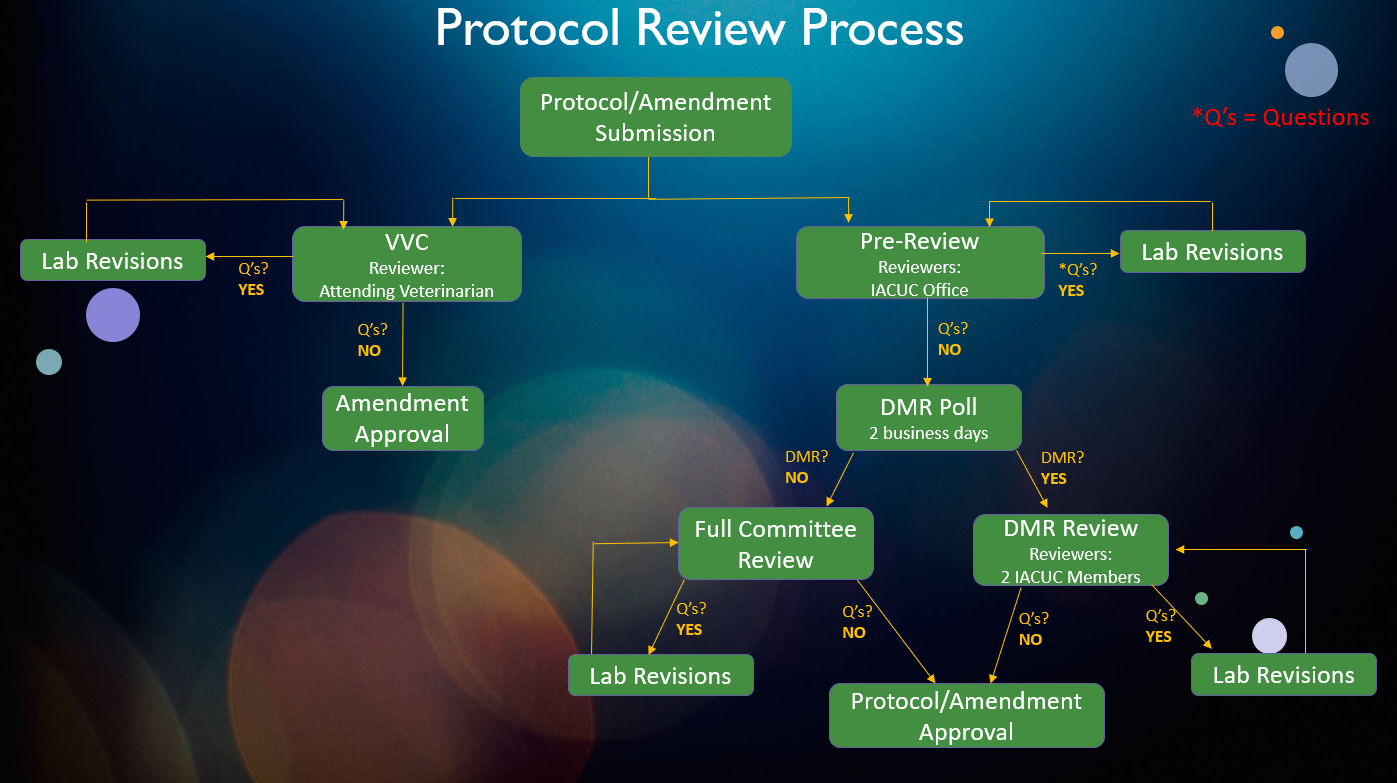
Please see the definitions tab for further details.
Definitions
- The IACUC Office checks all protocol submissions for completeness (i.e., all questions are answered), all documents are uploaded, and will send comments/questions.
- The protocol travels back-and-forth between the IACUC Office and the PI team until all identified issues are met.
- Veterinary verification and consultation is a method for confirming that changes to a previously approved protocol align with IACUC-approved guidance documents (e.g., drug formularies). It may not be used to add a new procedure that was not previously approved on the protocol. Such a change should be reviewed and approved by full committee review (FCR) or designated member review (DMR).
- All protocols/amendments that have passed the pre-review will be distributed to the entire IACUC as part of a DMR Poll. The DMR Poll gives the IACUC members a chance to quickly review the protocol/amendment and determine whether it should be reviewed by the Full Committee (FCR) or by Designated Members. DMR polls last 2 business days.
- If the IACUC members have indicated that the protocol/amendment can be reviewed through DMR, 2 IACUC members (typically the veterinarian and one other IACUC member) will be chosen as primary and secondary reviewers.
- The DMR members review the protocol and state the following options:
- Approve the protocol or amendment if all criteria are met
- Request revision(s) to the protocol or amendment if outstanding issues are present in order to secure approval
- Request FCR when further review of the protocol or amendment is warranted.
- The protocol travels back and forth between DMRs and the PI team with the IACUC administrator facilitating the exchange and maintaining confidentiality.
- FCR occurs during a convened meeting of the IACUC, held face-to-face (or electronically with real-time communication). Committee meetings are generally scheduled monthly on the third Wednesday. Changes do happen during holidays and when a need for a special meeting is requested.
- The following outcomes of a FCR may occur:
- Approval by a majority affirmative vote
- Revisions requested of the PI team to secure approval. Subsequent review may be done via DMR or FCR (as deemed appropriate at such meeting).
- Defer or table review of protocol if more clarification or outside consultation is needed.
- Withhold approval of protocol if it has not adequately addressed all requirements necessary.
- Investigators who desire to make modifications (also called amendments) to an approved
protocol during its approval period must submit a modification for approval prior
to initiating the change. Modifications include:
- Administrative changes such as addition or deletion of research personnel
- Significant changes such as modifications to the study design or objectives, the number of animals or species used, increases in pain or distress, changes to therapeutic or experimental agents or changes in euthanasia methods, and
- All others remaining. Approval of a modification does not alter the existing protocol's expiration date.
Protocol Review Timelines
Within 5 business days of submitting your protocol: confirmation from IACUC office that prereview is completed (and protocol is being sent to the IACUC members), or, some issues need resolution (before the protocol is ready for the IACUC members)
The following are the average timelines you can expect (to receive approval) for every
type of review:
Resources
- IACUC literature search guide at the Leatherby Libraries: https://chapman.libguides.com/iacuc
- USDA/APHIS information and Animal Welfare Act Regulations
- USDA’s policy 12 regarding “Consideration of Alternatives to Painful/Distressful Procedures”
- The AVMA Guidelines for the Euthanasia of Animals (2020 edition)
- American Society of Mammalogists IACUC, Guidelines for the capture, handling and care of mammals as approved by the American Society of Mammalogists
- Guidelines to the Use of Wild Birds in Research (2010)
- Use of Fishes in Research Committees, American Fisheries Society, Guidelines for the Use of Fishes in Research
- The Canadian Council on Animal Care, guidelines on the care and use of fish in research, teaching, and testing
- Herpetological IACUC of the American Society of Ichthyologists and Herpetologists, Guidelines for the Use of Live Amphibians and Reptiles in Field and Laboratory Research
- The Ag Guide, or the Guide for the Care and Use of Agricultural Animals in Research and Teaching