»Working with External Collaborators
There are several factors to consider when determining how to work on human participant research studies with non-Chapman personnel. Below are instructions on how and when to seek IRB approval when external collaborators are involved in your research. Download a PDF version of the Decision Tree with accessible links.
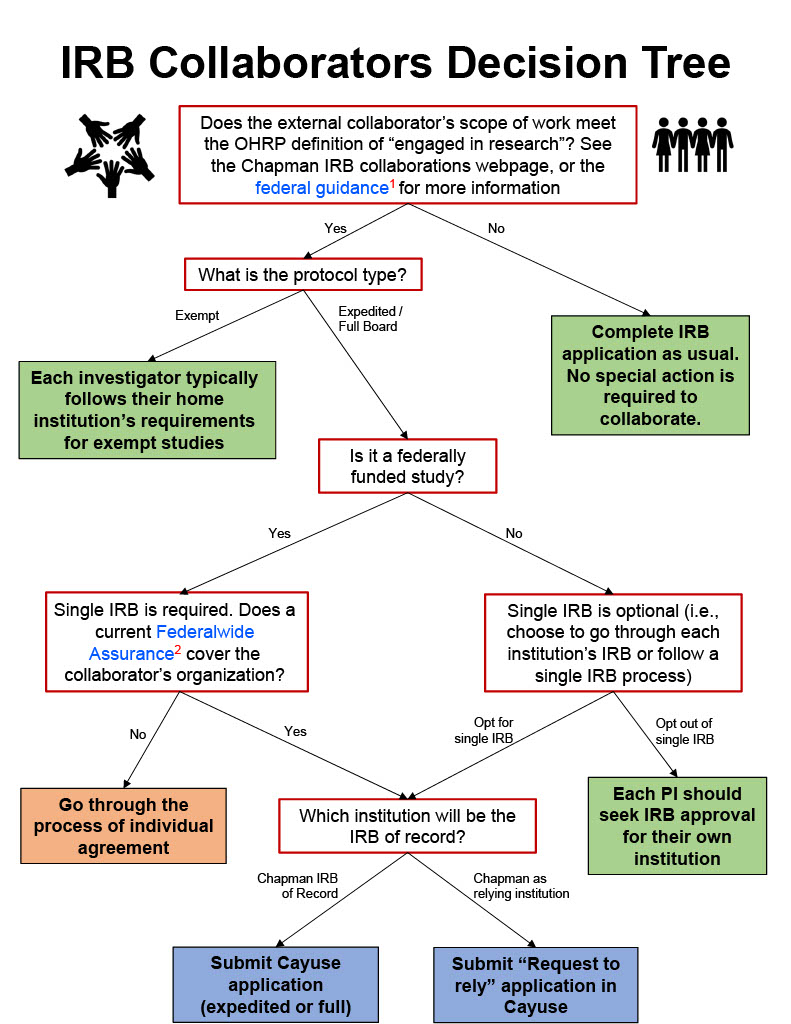
Are external collaborators “engaged in research”?
Does the external collaborator’s scope of work meet the OHRP definition of “engaged in research”? If the answer is “no,” the collaborator is not required to seek IRB approval for their involvement in the project.
An individual or an institution is engaged in the research when they will:
- obtain data about participants through intervention or interaction or manipulation of the participants’ environment.
- from any source, obtain, use, study, or analyze Identifiable private information or generate identifiable biological specimens from any source
- obtain informed consent from human participants for the research.
An individual is not engaged in research when their involvement is limited to:
- receiving and sharing IRB-approved recruitment materials (e.g., they are handing out flyers or forwarding an email) and will not answer questions about the study or obtain informed consent.
- receiving de-identified data, with no way to re-identify that data (e.g., through access to a code key or via indirect identifiers).
- the individual is a service-provider who will not be performing research or involved in the publication of study results.
Additional discussion on “engagement” in research can be found on the OHRP site, including information for contractors involved in IRB studies.
External collaborators do not need Chapman’s IRB approval for exempt studies
Is the study federally funded?
IRB collaboration agreements among institutions (i.e., Reliance or IRB Authorization Agreements "IAA")
One entity will conduct regulatory review of the study as the IRB of Record for all institutions which will be involved in the study. Each institution will enter into an IRB Authorization Agreement (IAA) with the IRB of Record so that the researchers from such institution may rely on the IRB of Record’s approval for the specific study.
Chapman may agree to be the IRB of Record when:
- Chapman received the prime award, and the project includes collaborators at other sites, e.g., subrecipients of funds from the prime award, and the Chapman IRB has the expertise to review the study.
- The Chapman principal investigator (PI) has adequate resources to support the compliant conduct of a multi-site study.
- The collaborating institution(s) have registered human subject research programs in good standing with the Office of Human Research Protections, including policies and procedures to oversee and take responsibility for the research. Search the database of approved institutions.
- The study is permitted under Chapman's Human Research Protection Program.
- The study undergoes either expedited or full board review. See above for exempt studies.
It is important to note that while a single IRB review helps avoid duplicate IRB committee reviews, it does not change the investigator's or the institution’s responsibilities for performing or overseeing the research. Therefore, Chapman and not its investigators will make the final determination on requests for either Chapman to serve as the IRB of Record or to rely on another IRB. See the instructions below for how to request the application through Cayuse.
Individual Investigator Agreement (IIA) for researchers unaffiliated with an institution with a current Federal wide Assurance (FWA)
In some cases, PIs may wish to engage individuals in their research (e.g., through a paid or unpaid collaboration agreement) where the individual is not associated with an institution that has a current FWA with the Office of Human Research Protections. In these cases, Chapman must agree to execute an Individual Investigator Agreement (IIA) before the individual may engage in research.
Individual Investigator Agreements may be appropriate when:
- The study is considered minimal risk, or more than minimal risks can be appropriately mitigated.
- The individual has the appropriate credentials to carry out the research and completes all training required by Chapman’s IRB.
- The Chapman PI will fill out the IIA, affix his/her/their signature along with that of the relying investigator, then upload such copy into Cayuse. Chapman’s Office of Research will obtain the signature of Chapman’s Institutional Official.
- The Chapman PI agrees to direct and appropriately supervise the individual’s activities.
- The individual agrees to the terms of the IIA.
To request an IIA:
- The external investigator must be listed in Cayuse as a researcher not affiliated with Chapman.
- Current CITI certificates for human subject protection courses are needed. The CITI courses are preferably completed with Chapman University as their affiliation. If CITI courses were completed under affiliation with another institution or an individual subscription, upload a copy of the CITI certificate and the course completion report showing the modules completed (if possible) into Cayuse.
Process: When Chapman relies on an external IRB
This section is used when Chapman cedes IRB review to an external institution (the
IRB of record).
An IRB reliance agreement (a.k.a. an IRB Authorization Agreement, "IAA") is required
when Chapman agrees to rely on an external institutions IRB for review of human subjects
research. In this case, the external institution will be the IRB of record. See
the Decision Tree above to determine when a single IRB may be appropriate. Submit
this application in Cayuse IRB only after you uploaded items 1 through 3 (of the list
below):
Notes:
Chapman is still responsible for oversight of the study involving its faculty, researchers, students, participants, or facilities, even if the study is approved by an external IRB. Throughout the life of the study, the Chapman PI is responsible for:
- Updating Chapman's IRB of changes in Chapman personnel
- Notifying Chapman's IRB of:
- Any changes in Chapman personnel
- Any changes in Chapman's role in the study
- Changes in the informed consent forms for the project
- Adverse events that involve Chapman personnel
- Changes in funding
- New/changes in potential conflicts of interest or commitment
Process: When an external institution relies on Chapman’s IRB
The Office of Research (OOR) will lead the process for Chapman to enter a Reliance Agreement that outlines the roles and responsibilities of each institution in the study. Chapman uses a template agreement that research institutions generally accept. OOR will work with the Chapman PI throughout the process and communicate any concerns or issues with the PI until the agreement is fully executed. Once the agreement has been fully executed, the initial submission or the modification will be approved in Cayuse IRB, and the OOR will issue formal approval of the research.
Reminder: Chapman PIs have the same responsibilities for overseeing studies and notifying the IRB of issues whether or not a Reliance Agreement is involved. Further, Chapman may also be responsible for informing a relying institution of issues, so be sure to notify the IRB of any issues.
- When the Relying Institution is included in a new study
Use this approach when Chapman and a Relying Institution plan to start the research simultaneously. Note that the IRB will approve the Chapman study only after the Reliance Agreement is fully executed with the collaborating institution(s).
- Log in to Cayuse IRB and complete the initial submission as usual. When prompted, choose "Other Researchers Not Affiliated with Chapman” in Section 2: Personnel.
- Provide the following information:
- The name of the external institution engaged in the research
- External institution’s currentFederalwide Assurance (FWA) number
- The name and email of the primary contact of the external institution’s IRB
- The name and email of the PI of the external institution
- The role of external investigators in the study
- The Local Context Form filled out and signed by the relying institution’s IRB representative. A copy of the form is in the IRB Forms webpage under “Research Collaborations”.
- OOR will review the information to determine whether Chapman’s criteria have been met. If such criteria are met, OOR will contact the relying institution to execute a reliance agreement. If the criteria are unmet or Chapman cannot execute a Reliance Agreement with the relying institution, OOR will inform the PI of such issues and discuss how to proceed.
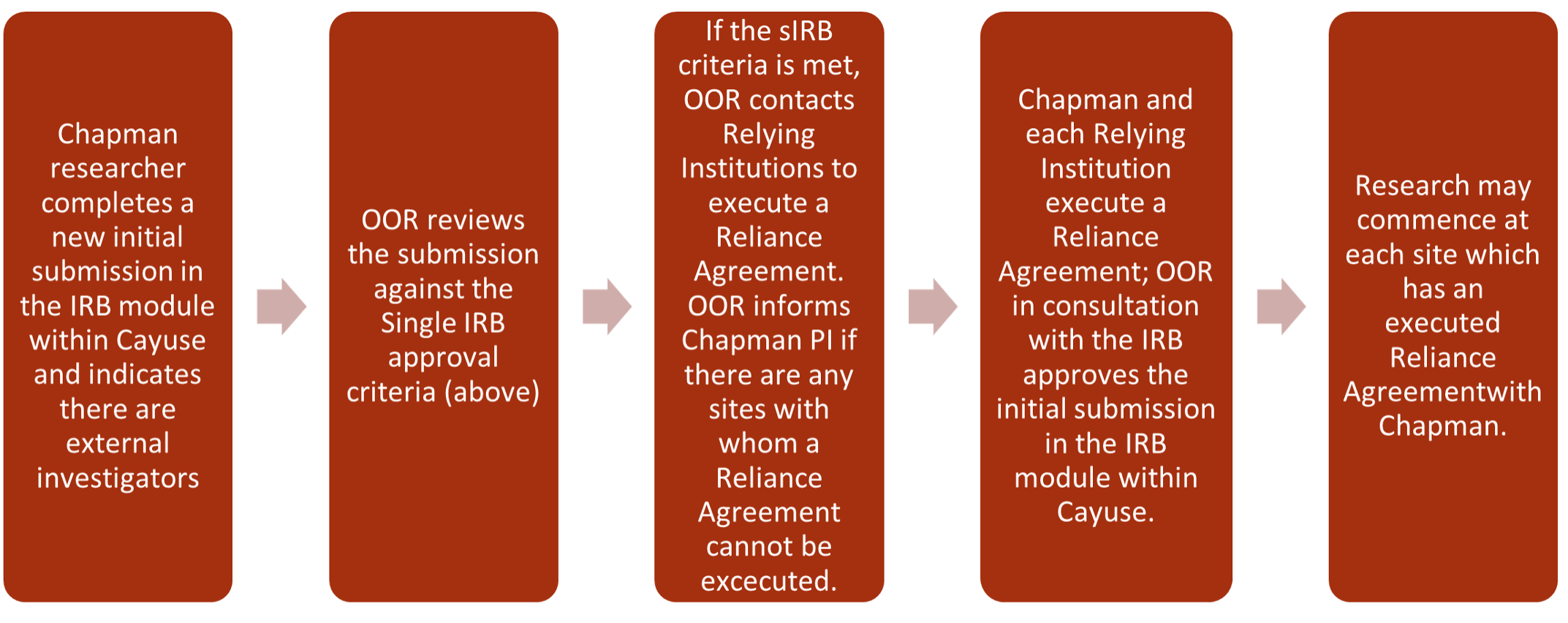
- Adding a relying institution to an IRB-approved Chapman study
- Log in to Cayuse IRB and start the Modification
- Provide the following information in Cayuse IRB:
- The name of the external institution engaged in research
- External institution’s active Federalwide Assurance (FWA) number
- The name and email of the primary contact of the external institution’s IRB
- The name and email of the PI of the external institution
- The role of external investigators in the study
- The Local Context Form completed by the external institution. A copy of the form is in the IRB Forms webpage under “Research Collaborations”.
- OOR will review the information to determine whether Chapman’s IRB criteria have been met. If such criteria have been met, OOR will contact each relying institution to execute a reliance agreement. If the single IRB criteria are not met, or the Reliance Agreement cannot be executed, the OOR will inform the PI of such issues and discuss how to proceed.

Processing the Agreements
The Office of Research (OOR) will lead the process for Chapman to enter a Reliance Agreement that outlines the roles and responsibilities of each institution in the study. Chapman uses a template agreement that research institutions generally accept. OOR will work with the Chapman PI throughout the process and communicate any concerns or issues with the PI until the agreement is fully executed. Once the agreement has been fully executed, the initial submission or the modification will be approved in Cayuse IRB, and the OOR will issue formal approval of the research.
Reminder: Chapman and PIs have the same responsibilities for overseeing studies and notifying the IRB of issues whether or not a Reliance Agreement is involved. Further, Chapman may also be responsible for informing a relying institution of issues, so be sure to notify the IRB of any issues.
Moreover, once a Reliance Agreement has been approved, Chapman researchers are responsible for initiating modifications for any changes in the project (e.g., changes in Chapman personnel) following the usual IRB process.
Definitions
- IRB of Record: The IRB is responsible for the ethical review of human research on behalf of an institution, site, or individual investigator.
- Relying Institution: An institution that has agreed to cede review to an external IRB for a particular study.
Frequently Asked Questions
- How long will it take?
Reliance agreements can be executed relatively quickly (about two weeks) for minimal risk studies with a partner that Chapman works with regularly. Alternatively, they can take up to two months for complex studies or new Relying Institutions. PIs are urged to build in additional time for this process to allow for unanticipated delays.
- What’s the policy when Chapman researchers are the only participants in a study approved at an external institution and have an appointment at the external institution (e.g., they have an unpaid collaboration appointment)? Does the single IRB process apply?
If the faculty PI’s primary appointment is at Chapman, or if anyone at Chapman is involved in the study as a participant or researcher (e.g., other faculty PIs, students, postdocs), then the study should also be reviewed by Chapman. Use the process for relying on another institution’s IRB.
3.What if a researcher has part-time or joint appointments at both institutions in a research study? At which institution should the person be listed?
Part-time employees should rely on the IRB at their primary employer (where there is a greater level of effort or a more senior role). Chapman’s IRB would still review the study when its employees or students are involved. This is true whether or not Chapman is IRB of Record. In instances when another institution, Chapman’s IRB needs to ensure that internal policies and requirements are still being met.