The mission of the Center for Targeted Drug Delivery (CTDD) is to design, develop, and evaluate novel approaches for targeted delivery of therapeutic agents. Our vision for the research conducted by our members is to improve the efficacy and safety of therapeutic agencies through innovative technologies.

» Center for Targeted Drug Delivery
Partnership Interest Form
The Center for Targeted Drug Delivery (CTDD) is seeking academic, industry and international partners for collaboration in our dynamic, innovative research.
For more information on this exciting opportunity with Chapman's CTTD, please fill out this form »
Structural Biology Research Center (SBRC)
The Structural Biology Research Center (SBRC) is housed by the Chapman University School of Pharmacy (CUSP) in the Harry and Diane Rinker Health Science Campus, Irvine. The center offers expertise and access to cutting-edge instrumentation for biomolecular structural analysis and drug development, including x-ray crystallography, NMR spectroscopy, and protein-ligand interaction analysis.
Our mission at SBRC is to advance structure-based drug design and development by integrating of structural and biophysical techniques for the characterization of the structure and function of biomolecules and macromolecular complexes. To accomplish this mission, we aim to develop collaborations and partnerships with other academic institutions as well as Biotech and pharmaceutical companies.
Center Activities:
SBRC provides training, access to the equipment, and any needed assistance in sample preparation, as well as X-ray diffraction, NMR, and SPR data collection and analysis.
SBRC Members:
- Dr. Innokentiy Maslennikov - NMR Spectroscopy
- Dr. Miao Zhang - X-ray Crystallography
- Dr. Gennady Verkhivker - Computational Biology
Equipment
X-ray Crystallography
 Mosquito Crystallization Robot (TTP Labtech. Inc)
Mosquito Crystallization Robot (TTP Labtech. Inc)
- High-throughput crystallization of biomacromolecules (soluble and membrane proteins, peptides)
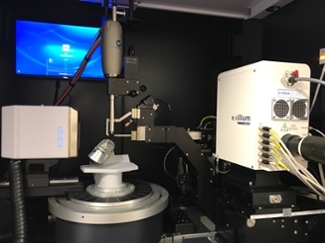 D8 Venture Liquid Gallium Metaljet (Bruker)
D8 Venture Liquid Gallium Metaljet (Bruker)
- X-ray diffraction data collection
- X-ray diffraction data analysis and structure determination
Stereo Microscope

Nuclear Magnetic Resonance (NMR)
 Avance III HD 400 MHz (Bruker)
Avance III HD 400 MHz (Bruker)
- Solution NMR spectroscopy data collection (1H, 13C, 15N, 19F, 31P, etc.)
- Small Molecule/Protein Characterization
- Label-free detection of ligand-target interactions
Protein-Ligand Interaction Assay
Biacore S200 (GE Healthcare)
- Surface Plasmon Resonance (SPR)
- Label-free detection of protein-protein or protein-ligand interactions
SBRC Pricing
For information regarding SBRC pricing, please click here to learn more.